The DESWARE meets a long-standing need in the desalination and water resources community for a detailed archival source of knowledge.

The conversion of saline water is a technique of providing and augmenting freshwater supplies in areas deficient therein

The conversion of saline water is a technique of providing and augmenting freshwater supplies in areas deficient therein

DESWARE is a stand-alone encyclopedia focusing on the specific topics of desalination and water resources developed in parallel with the main EOLSS project.
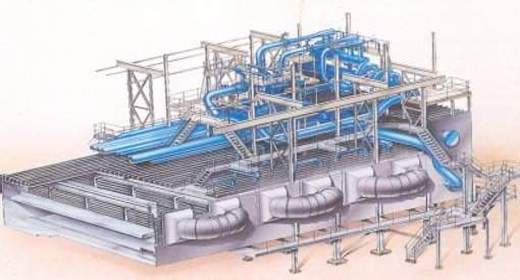
This new generation of MSF evaporators incorporates significant developments in material technology and process control optimisation.

Sun is the Source of Renewable Energy and the Oceans are a Major Alternative Source of Water

Human engineered desalination systems actually mimic the hydrologic cycle, which is itself a grand process of distillation
DESWARE is a subset of the Encyclopedia of Water Sciences, Engineering and Technology Resources, which is part of the UNESCO Encyclopedia of Life Support Systems (EOLSS)
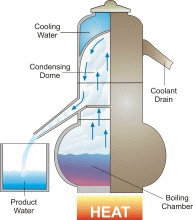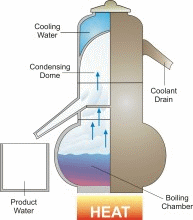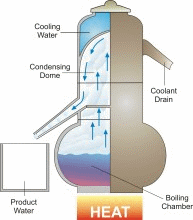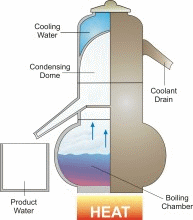
Distillation is one of mankind’s earliest forms of separating fresh water from a salt-water solution. When salt water is boiled, the dissolved salt remains behind as the fresh water vapor is boiled away. In a distillation process, water is first boiled and then the steam, or water vapor, is cooled. This cooling condenses the steam into water again (See the figure). Thus, distillation involves adding heat energy to salt water in order to vaporize the water and then removing the heat energy from the steam to condense it into fresh water.
In nature, this basic process is responsible for the hydrologic cycle. The sun causes water to evaporate from surface sources such as lakes, oceans, and streams. The water vapor eventually comes in contact with cooler air, where it re-condenses to form dew or rain. This process can be imitated artificially, and more rapidly than in nature, using alternative sources of heating and cooling.
When water is heated, its temperature increases until the
boiling point is reached. While water is boiling, the
steam and the boiling water are at the same temperature.
However, raising water to its boiling point is not enough to
cause it to boil. More heat must be added to change the water
into steam. The amount of heat required to change water at its
boiling point into steam at the same temperature is called heat
of vaporization of water. The heat of vaporization is of major
importance in distillation. The amount of heat required to
vaporize water into steam is approximately five times greater
than the heat needed to raise water from its freezing point to
its boiling point (at ordinary sea-level atmospheric pressure
(14.7 psi) water boils at 100oc).
Distillation is a two-step process involving both evaporation
and condensation, heat must be added in one step and removed in
the other. If these two steps were accomplished independently,
the process would be inefficient and costly. In all the
distillation processes, the steam is condensed by transferring
heat from the steam to salt water as part of the heat source
required to convert more water into steam. In this way some of
the heat energy used in one step is recovered and used in the
other step.
The conversion of saline water into fresh potable water and water for industrial purposes has been practiced worldwide over the last forty to fifty years of the twentieth century. It is a technique of providing and augmenting freshwater supplies in areas deficient therein, such as arid regions close to the ocean, or to other saline water bodies. Read more..
Water has always been earth's most valuable resource. All ecosystems and every field of human activity depend on water. The world's supply of fresh water is running out. Already one person in five has no access to safe drinking water. The amount of water in the world is limited. The human race, and the other species which share the planet, cannot expect an infinite supply. 97.5% of the total global stock of water is saline and only 2.5% is fresh water. Approximately 70% of this global freshwater stock is locked up in polar icecaps and a major part of the remaining 30% lies in remote underground aquifers.
Population growth and the increasing need for fresh water for industrial, agricultural uses and municipal indicate there will be no letup in the increasing demand for water in the years to come. These factors account for the concern over water shortages that exist now in some areas of the country and over the more serious shortages that are projected for the near future.
See : Desalination and the Continuity of Human Civilization

1- Dead vegetation in drought-stricken area, Senegal.

2- Children have a special relationship with water (Source: UNESCO Photo bank)
DESWARE is focusing on the specific topics of desalination and water resources. The encyclopedia presents a detailed and comprehensive coverage of the interdisciplinary subjects like science, technology, and management experience available for the design, operation, and maintenance of desalination plants. Explore